
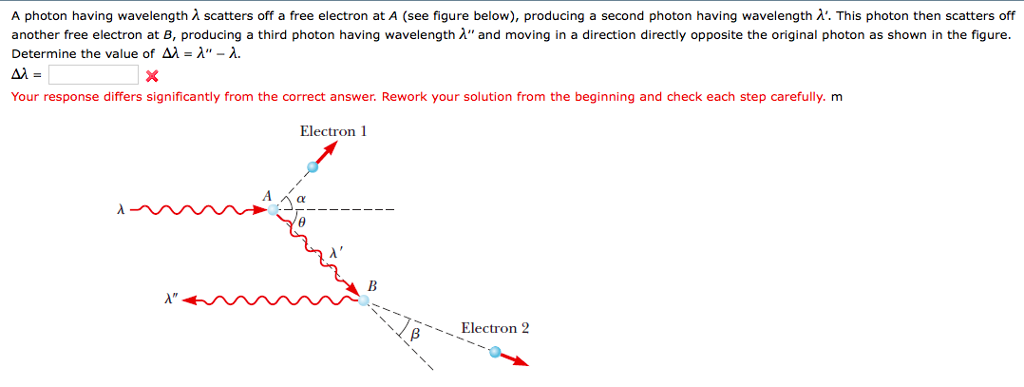
The complete list of calculated photon ionization energies from hydrogen to calcium, for all orbitals, is here.Įxample Calculation – Photon Wavelength Hydrogen – Orbital Transition from 3s -> 2s These calculations are placed in the Atoms section of this web site after an explanation and calculation of electron distances. The photon energies for orbitals beyond the 1s orbital requires the electron distance to be known. Ionization Energy of Hydrogen to Calcium – All Orbitals This equation only applies to the 1s orbital.* Instead, the Bohr radius is used as the orbital distance when this equation is used. In these calculations, the Amplitude Factor Equation – 1s Orbital Ionization is used to solve one variable without knowing orbital distance. A pattern emerges for the first orbital of elements from hydrogen to calcium that allow a simplified method reducing two variables to one. In this section, the photon energy of the 1s electron is described. For example, as the electron transitions from the 3s orbital to 2s orbital (3->2) it emits a photon calculated to be 6.56E-07 meters.Ĭalculations – Photon Energy Ionization Energy of the 1s Electron – Neutral Element (from Spectroscopy Experiments) Calculations are compared to measured results for photons wavelengths of hydrogen. Using the photon wavelength equation, the wavelengths of photons emitted during hydrogen for an electron transitioning from an excited state (orbitals 3, 4, 5, 6, 7, 8, 9) to the second (2) orbital. Using the photon wavelength equation, the wavelengths of photons absorbed during hydrogen ionization were calculated for the ground state and each of the excited states (orbitals 1, 2, 3, 4, 5, 6).
Calculations – Photon Wavelengths Hydrogen Wavelengths (Ionization)


 0 kommentar(er)
0 kommentar(er)
